Introduction
The use of venetoclax in acute myeloid leukemia (AML) has become routine - following FDA approval, approximately half of patients with AML have received venetoclax. However, the outcomes of the timing of venetoclax initiation remain unknown. Such outcomes are of notable importance, as it is often critical to allow time for patient optimization and to await molecular profiling - particularly as ongoing research contributes to a deeper understanding of the mechanisms of venetoclax sensitivity and resistance. A critical real-world limitation is that the turnaround times of complete molecular profiling via NGS assays are frequently 14 to 21 days. Therefore, we performed the first stratified analysis that details the impact of delays in venetoclax-based regimen initiation on overall survival.
Methods
We analyzed 185 patients with AML treated with venetoclax and a hypomethylating agent (azacitidine or decitabine; VEN+HMA) from January 1, 2018 to April 18, 2023 at VCU Massey Comprehensive Cancer Center. We recorded baseline patient-related and disease characteristics, including age, ECOG, Charlson comorbidity index (CCI) scores, molecular profiling and ELN 2022 cytogenetic risk, dates of regimen initiation, and survival. We individually analyzed the first-line and relapsed or refractory (RR) groups. In each group, we separated patients into three cohorts: those that started therapy in 0-7 days, 8-14 days, and 15 days and beyond. We used the D'Agostino & Pearson method for normality testing and the Mann-Whitney test for between-group comparisons. We applied the Bonferroni correction if multiple comparisons were made. We analyzed survival by the Kaplan-Meier method, with significance determined by the log-rank test. The event for calculating the overall survival (OS) was the date of death. Patients were otherwise censored at the date of last contact.
Results
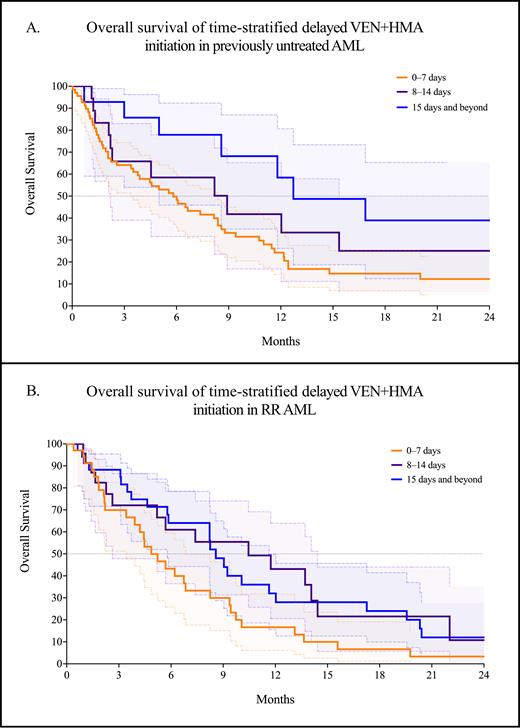
Of the 185 patients treated with venetoclax and a hypomethylating agent, 100 (54.1%) were in the first-line setting, and 85 (45.9%) had RR AML. In the first-line setting, the median time to initiation of VEN+HMA was 5 days (range: 0-69 days). Compared with the first-line setting, VEN+HMA for RR AML was started at a significantly longer time from the date of disease progression at a median of 11.5 days (range: 0-110 days, p < 0.0001). In the first-line setting, the median OS was significantly worse for earlier initiation of VEN+HMA at 5.8 months for the 0-7 day cohort, compared with 8.9 months for the 8-14 day cohort and 12.7 months for 15 days and beyond (p = 0.023, Figure A). Similarly, in RR AML, the median OS was also significantly worse in the 0-7 day cohort at 4.9 months - but significantly better in the 8-14 day cohort at 10.5 months compared with the 15-day and beyond cohort at 7.1 months (p = 0.027, Figure B).
Discussion
In the first-line setting, short delays in initiating VEN+HMA do not adversely impact survival. This approach may be preferred to optimize comorbidities and disease-related complications while awaiting the results of molecular profiling. In RR AML, delays in therapy initiation also do not appear to decrease survival - although the optimal therapeutic window appears to be shorter. Taken together, these findings are the first to suggest that with lower-intensity venetoclax-based strategies, short delays in treatment for patient optimization and comprehensive molecular profiling are associated with improved survival.
Disclosures
Grant:Prescient Therapeutics: Research Funding. Maher:Sobi (Doptelet): Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal